Oil Refining and Gas Processing
Turning complex mixtures into usable products
Introduction
Crude oil and natural gas are complex chemical mixtures that are generally unsuitable for direct use. Oil refining and gas processing turn these mixtures into a wide range of fuels and other products while removing low-value and polluting components.
Refining and processing have both positive and negative environmental impacts: although they remove harmful pollutants and produce cleaner-burning fuels, the operations at refineries and processing plants may release harmful pollutants into the environment, affecting local air and water quality.
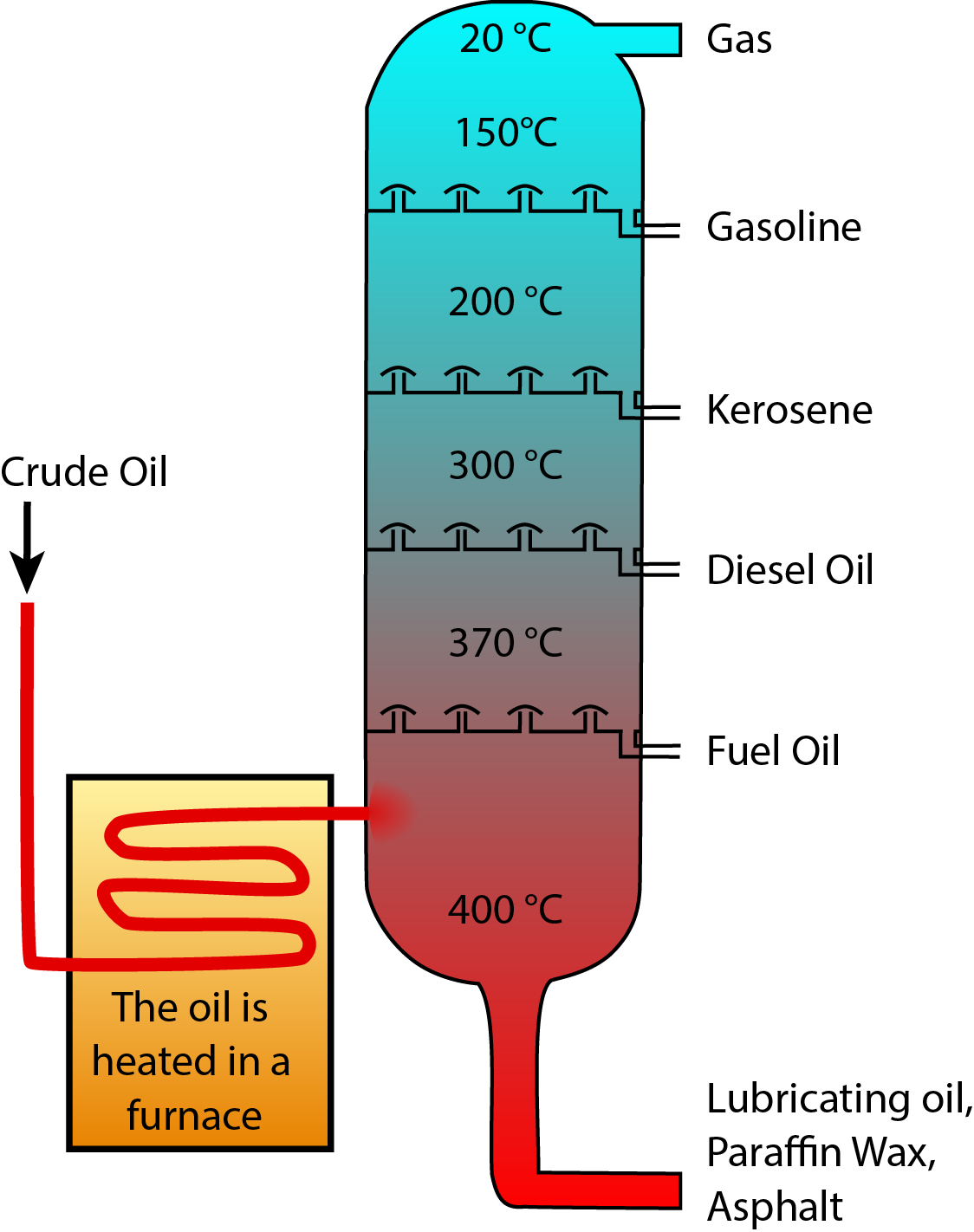
During crude oil distillation, different fuel types condense and are extracted at different temperatures. Image Credit: Wikimedia Commons users Psarianos and Theresa Knott.1
Oil Refining
Crude oil is a mixture of many different hydrocarbon molecules of a range of sizes. Smaller molecules vaporize at lower temperatures, so crude oil can be distilled to separate out the different hydrocarbons. In the distillation process, crude oil is vaporized and the hot vapor rises up a column, cooling as it rises. Different hydrocarbons vaporize at different temperatures, so they condense into liquid form at different points in the column, separating the crude oil into different components that can then be further processed to optimize them for their final use.
Gasoline and diesel are the most lucrative products extracted from crude oil, so refineries use a range of techniques to maximize the production of these fuels. This may include cracking (breaking larger molecules down into smaller molecules2), hydrotreating (replacing impurities such as sulfur with hydrogen to improve fuel quality3), reforming (turning smaller molecules into gasoline2), alkylation (using an acid to produce high-octane gasoline from smaller molecules4), and blending (mixing different liquids together to produce uniform products that meet regulatory standards5). During the blending stage, ethanol from industrial ethanol plants is also blended into gasoline to increase its octane content, reduce carbon monoxide emissions, and meet the requirements of the Renewable Fuel Standard.6
Products of Oil Refining
Different crude oils have different compositions, containing different mixtures of hydrocarbons and variable amounts of sulfur and other impurities. The proportions of different refined products will vary with changes in the types of oil being refined, demand for different products, and regulations that influence this demand. Roughly 80-85% of all crude oil ends up as gasoline, diesel, or jet fuel. The rest is used to produce liquefied petroleum gases, petrochemical feedstocks, and a variety of other products.7 In 2016, 141 U.S. refineries produced a daily average of 9.3 million barrels of gasoline, 3.7 million barrels of low-sulfur diesel, and 1.6 million barrels of jet fuel.8
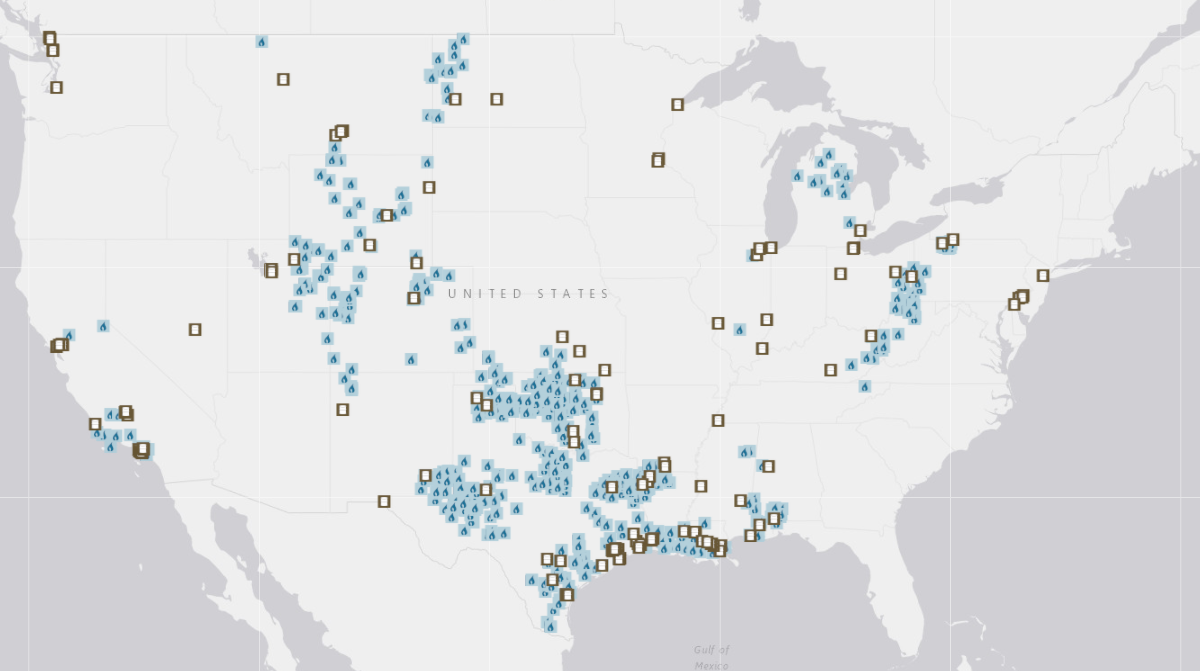
Oil refineries (open squares) and gas processing plants (blue) in the United States as of February 2018. Not shown: two refineries in Hawaii and five in Alaska. Image credit: U.S. Energy Information Administration.17
Natural Gas Processing
In 2017, the United States produced 33 trillion cubic feet of natural gas.9 A small fraction of this was used in field operations, re-injected into underground reservoirs, vented, or flared; the rest was processed by 550 gas processing plants to produce 27 trillion cubic feet of pipeline-quality natural gas.10,11 Pipeline-quality gas must meet rigid standards for energy content and purity12 for residential, commercial, and industrial use, including natural gas power plants.
Before processing, natural gas consists mostly of methane, with varying proportions of other hydrocarbons, carbon dioxide (CO2), sulfur dioxide, nitrogen, water vapor, and helium.13 Gas processing removes some of the non-methane components of natural gas in order to:
- Improve combustion and reduce corrosion by removing water
- Prevent the formation of damaging acids by removing harmful or corrosive gases – especially sulfur and CO2 – that might otherwise react with small amounts of water to form acids
- Standardize the energy content of the gas to ensure uniform combustion in furnaces and other equipment, notably by removing non-combustible gases such as CO2 and nitrogen
- Extract valuable minor gases for other uses (e.g., other hydrocarbons and helium)
Non-methane hydrocarbons extracted during gas processing are collectively called “natural gas liquids” (NGLs) because they form liquids more easily than methane at high pressure or low temperature. Of the NGLs, the most common are ethane, propane, and butane. Ethane and propane are further processed in large quantities to make feedstocks for plastics (see “Non-Fuel Products of Oil and Gas” in this series), while propane and butane are compressed into liquids to provide an energy-dense source of gas fuel for off-grid uses.
The main methods used to remove non-methane components from natural gas are absorbents and cooling. A variety of absorbents may be used, including special oils (for NGLs), glycol (for water), amines (for sulfur and CO214), and zeolite or oil absorption (for nitrogen15). Chilling natural gas down to different temperatures allows different components to be removed as they condense into liquids. This is the most common method for nitrogen removal: the natural gas is chilled until the methane liquefies, allowing the nitrogen gas to be vented off.16 NGLs may be removed in a single mixture that is then heated to different temperatures to isolate each NGL in turn.18 After processing, the gas is deemed “dry” and ready to be shipped via pipelines to end users.
Refining, Processing, and the Environment
Refining and processing reduce the environmental impact of oil- and gas-derived fuels by removing harmful pollutants and improving their reliability during combustion. However, refineries and processing plants have their own environmental impacts, with corresponding procedures for minimizing those impacts. More information on these can be found in other parts of this series: “Mitigating and Regulating Methane Emissions” and “Air Quality Impacts of Oil and Gas.”
Carbon dioxide (CO2) occurs in varying proportions in natural gas and is removed at processing plants to improve the quality of the gas. Most of this CO2 is vented to the atmosphere, accounting for roughly 0.4% of total U.S. greenhouse gas emissions (for comparison, methane leaks from the natural gas production and distribution chain are estimated to account for roughly 3% of U.S. emissions).19 A small number of gas processing plants capture the CO2 removed from natural gas during processing; this captured CO2 is injected into oil fields to enhance oil recovery.20
References
1 File:Crude Oil Distillation-en. Wikimedia Commons users Psarianos & Theresa Knott. Reproduced according to a CC BY-SA 3.0 license. (https://creativecommons.org/licenses/by-sa/3.0/deed.en)
Accessed from: https://commons.wikimedia.org/wiki/File:Crude_Oil_Distillation-en.svg
2 Centre for Industry Education Collaboration, University of York (2014). Cracking and related refinery processes. The Essential Chemical Industry – online.
Accessed from: http://www.essentialchemicalindustry.org/processes/cracking-isomerisation-and-reforming.html
3 Kokayeff, P. et al. (2014). Hydrotreating in Petroleum Processing. In: Treese, S., Jones, D., Pujado, P. (eds). Handbook of Petroleum Processing. Springer, Cham.
Accessed from: https://link.springer.com/referenceworkentry/10.1007%2F978-3-319-05545-9_4-1
4 U.S. Energy Information Administration (2013). Alkylation is an important source for octane in gasoline. Today in Energy, February 13, 2013.
Accessed from: https://www.eia.gov/todayinenergy/detail.php?id=9971
5 U.S. Environmental Protection Agency – Gasoline Standards: Gasoline Reid Vapor Pressure.
Accessed from: https://www.epa.gov/gasoline-standards/gasoline-reid-vapor-pressure
6 U.S. Energy Information Administration – Biofuels: Ethanol and Biodiesel Explained – Use of Ethanol.
Accessed from: https://www.eia.gov/energyexplained/index.cfm?page=biofuel_ethanol_use
7 U.S. Energy Information Administration – Oil: Crude and Petroleum Products Explained – Refining Crude Oil.
Accessed from: https://www.eia.gov/energyexplained/index.cfm?page=oil_refining
8 U.S. Energy Information Administration – Petroleum & Other Liquids: U.S. Product Supplied, Total Crude Oil and Petroleum Products.
Accessed from: https://www.eia.gov/dnav/pet/pet_cons_psup_dc_nus_mbblpd_a.htm
9 U.S. Energy Information Administration – U.S. Natural Gas Gross Withdrawals.
Accessed from: https://www.eia.gov/dnav/ng/hist/n9010us2a.htm
10 U.S. Energy Information Administration – Natural Gas Annual Respondent Query System, EIA-757: Natural Gas Processing Capacity by Plant , Data through 2014.
Accessed from: https://www.eia.gov/cfapps/ngqs/ngqs.cfm?f_report=RP9
11 U.S. Energy Information Administration – U.S. Dry Natural Gas Production.
Accessed from: https://www.eia.gov/dnav/ng/hist/n9070us2A.htm
12 North American Energy Standards Board.
Accessed from: https://naesb.org/
13 Penn State College of Earth and Mineral Sciences, e-Education Institute – Petroleum Processing: Natural Gas Composition and Specifications.
Accessed from: https://www.e-education.psu.edu/fsc432/content/natural-gas-composition-and-specifications
14 Rufford, T.E. et al. (2012). The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng., 94-95, 123-154.
Accessed from: https://www.sciencedirect.com/science/article/pii/S0920410512001581
15 Sep-Pro Systems – Nitrogen Rejection Units.
Accessed from: http://www.sepprosystems.com/Nitrogen_Rejection_Units.html
16 U.S. Energy Information Administration (2006). Natural Gas Processing: The Crucial Link between Natural Gas Production and Its Transportation to Market.
Accessed from: http://www.dnr.louisiana.gov/assets/docs/oilgas/naturalgas/ngprocess_20060131.pdf
17 U.S. Energy Information Administration – U.S. Energy Mapping System.
Accessed from: https://www.eia.gov/state/maps.php
18 U.S. Department of Energy (2017). Natural Gas Liquids Primer, with a Focus on the Appalachian Region.
Accessed from: https://www.energy.gov/sites/prod/files/2017/12/f46/NGL%20Primer.pdf
19 U.S. Environmental Protection Agency (2017). Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2015.
Accessed from: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2015
20 Global CCS Institute – Projects Database: Large-scale CCS facilities.
Accessed from: https://www.globalccsinstitute.com/projects/large-scale-ccs-projects
Table of Contents
- #1: Petroleum and the Environment: an Introduction
- #2: Water in the Oil and Gas Industry
- #3: Induced Seismicity from Oil and Gas Operations
- #4: Water Sources for Hydraulic Fracturing
- #5: Using Produced Water
- #6: Groundwater Protection in Oil and Gas Production
- #7: Abandoned Wells
- #8: What Determines the Location of a Well?
- #9: Land Use in the Oil and Gas Industry
- #10: The Pinedale Gas Field, Wyoming
- #11: Heavy Oil
- #12: Oil and Gas in the U.S. Arctic
- #13: Offshore Oil and Gas
- #14: Spills in Oil and Natural Gas Fields
- #15: Transportation of Oil, Gas, and Refined Products
- #16: Oil Refining and Gas Processing
- #17: Non-Fuel Products of Oil and Gas
- #18: Air Quality Impacts of Oil and Gas
- #19: Methane Emissions in the Oil and Gas Industry
- #20: Mitigating and Regulating Methane Emissions
- #21: U.S. Regulation of Oil and Gas Operations
- #22: Health and Safety in Oil and Gas Extraction
- #23: Subsurface Data in the Oil and Gas Industry
- #24: Geoscientists in Petroleum and the Environment
- Glossary of Terms
- Full Reference List
